Surgical Clothing and Drapes
Performance requirements for surgical clothing and drapes
Surgical drapes, including the intended use as a sterile field, and surgical gowns are used to minimize the spread of infective agents to and from patients’ operating wounds, thereby helping to prevent postoperative wound infections
Resistance to wet bacterial penetration, ISO 22610
This test determines the resistance of a material to the penetration of bacteria from a dry surface through a material by the combined effect of friction, pressure and wetting . The pressure is intended to mimic the type of pressure exerted by a surgeon's elbow during a procedure and was developed specifically to measure the penetration by bacteria through operation materials of reusable or single-use material.
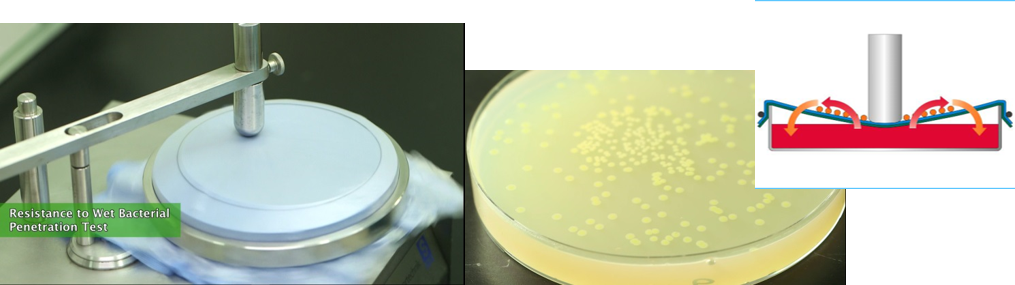
Resistance to dry bacterial penetration, ISO 22612
Dry bacterial penetration is a test method that was designed to simulate the penetration of bacteria-carrying skin scales through fabrics. This test provides a means for assessing the resistance to penetration through barrier materials of bacteria-carrying particles.

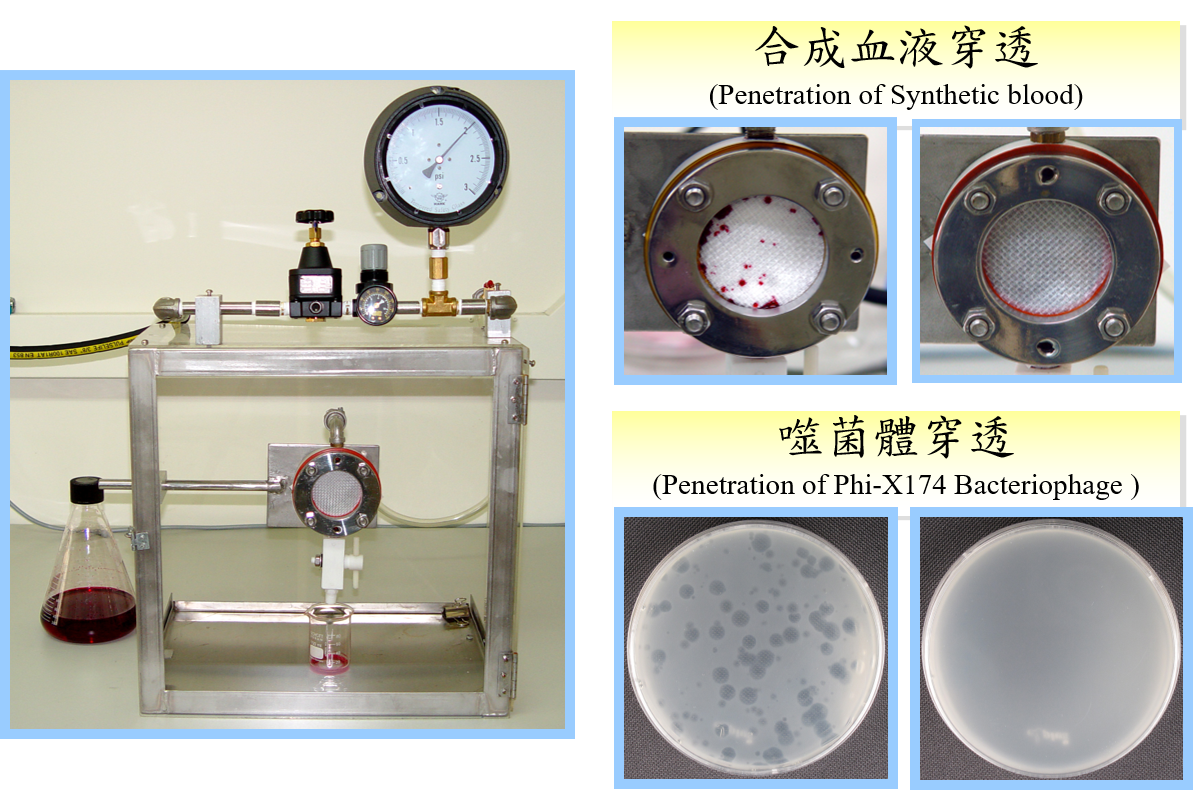
Synthetic Blood / Viral Penetration for Liquid Barriers, ASTM F1670 (ISO 16603) / ASTM F1671 (ISO 16604)
The test method is used to evaluate the resistance of materials used in medical protective textiles to penetration by synthetic blood or blood-borne pathogens using Phi-X174 bacteriophage under conditions of continuous liquid contact.
BS EN 13795:2019 Performance requirements for surgical clothing and drapes
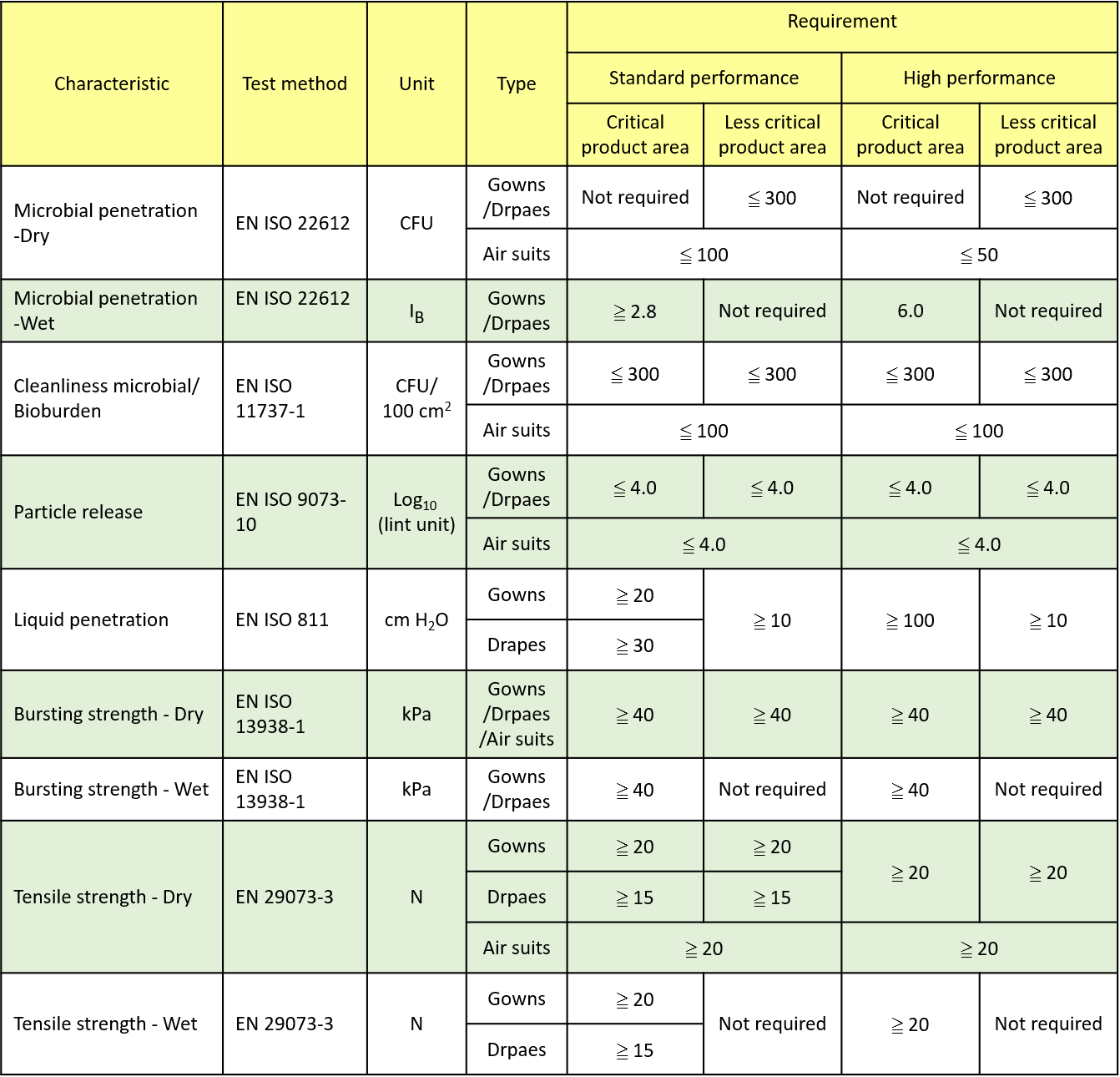
ANSI/AAMI PB70:2012 Performance requirements for protective apparel and drapes
Related Standards:
BS EN 13795:2011+A1:2013 Surgical drapes, gowns and clean air suits, used as medical devices for patients, clinical staff and equipment. General requirements for manufacturers, processors and products, test methods, performance requirements and performance levels
BS EN 14126:2003 Protective clothing. Performance requirements and tests methods for protective clothing against infective agents
ANSI/AAMI PB70:2012 Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities
ASTM F2407-06(2013)e1 Standard Specification for Surgical Gowns Intended for Use in Healthcare Facilities
ISO 22610:2018 Surgical drapes, gowns and clean air suits, used as medical devices, for patients, clinical staff and equipment -- Test method to determine the resistance to wet bacterial penetration
ISO/DIS 22611:2003 CLOTHING FOR PROTECTION AGAINST INFECTIOUS AGENTS - TEST METHOD FOR RESISTANCE TO PENETRATION BY BIOLOGICALLY CONTAMINATED AEROSOLS
ISO 22612:2005 Clothing for protection against infectious agents -- Test method for resistance to dry microbial penetration
ASTM F1670/F1670M-17a Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Synthetic Blood
ISO 16603:2004 Clothing for protection against contact with blood and body fluids — Determination of the resistance of protective clothing materials to penetration by blood and body fluids — Test method using synthetic blood
ASTM F1671/F1671M-13 Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System
ISO 16604:2004 Clothing for protection against contact with blood and body fluids — Determination of resistance of protective clothing materials to penetration by blood-borne pathogens — Test method using Phi-X 174 bacteriophage