Sterilizing Filtration of Liquids
Sterilizing filtration is a critical step in an aseptic manufacturing process. Validation of sterilizing filtration processes can be complex and is generally conducted in both a process and product specific manner. This document describes requirements that, if met, will provide a sterilizing filtration process that consistently removes microorganisms from a fluid (liquid or gas) without negatively affecting the quality of the filtrate. Furthermore, conformity with the requirements ensures that a sterilizing filtration process is both reliable and reproducible so that a determination can be made, with reasonable confidence, that the sterilizing grade filter/s will provide a sterile filtrate under specified operational conditions. This (the reliability and reproducibility of the filtration process) is essential, as unlike a micro-biocidal sterilization process where process variables can be monitored continuously, microbial retention and physical integrity of a sterilising grade filter cannot be monitored on a continuous basis throughout a filtration process.
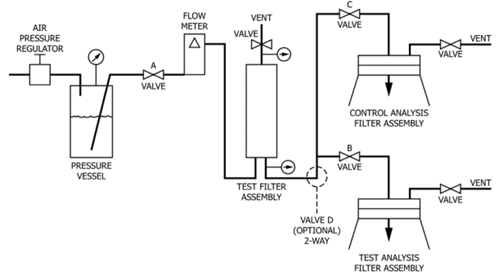
The test method of ASTM F838 determines the bacterial retention characteristics of membrane filters for liquid filtration using Brevundimonas diminuta as the challenge organism. This test method may be employed to evaluate any membrane filter system used for liquid sterilization.
After sterilization, the test filter is challenged with a suspension of B. diminuta (ATCC 19146) at a concentration of 107 organisms per cm2 of effective filtration area (EFA) at a maximum differential pressure across the test filter of 30 psig (206 kPa) and a flow rate of 2 to 4 × 10–3 LPM per cm2 of effective filtration area. The entire filtrate is then filtered through an analytical membrane filer disc, which is subsequently incubated on a solidified growth medium. Microorganisms that are not retained by the filter being tested will develop into visible colonies on the analysis membrane and can then be enumerated.
Applicable Standards:
- ASTM F838
- PDA Technical Report 26
- AAMI/ISO 13408